- → Biofilter
- → Bioscrubber
- → Biotrickling filter
- → Water scrubber
- → Acid scrubber
- → Caustic scrubber
- → Oxidative scrubber
- → Reductive scrubber
- → Solvent scrubber
- → Liquid jet gas ejector scrubber
- → Gas jet liquid ejector scrubber
- → Venturi scrubber
- → Wet dust separator
- → Bubble reactor
- → Dry scrubber
- → Dispersion fan
- → Stripper
Application
Removal of volatile compounds from a waste water flow:
chlorinated hydrocarbons
aromatic compounds in soil remediation
others (NH3, H2S, ethylene oxide, CO2, …)
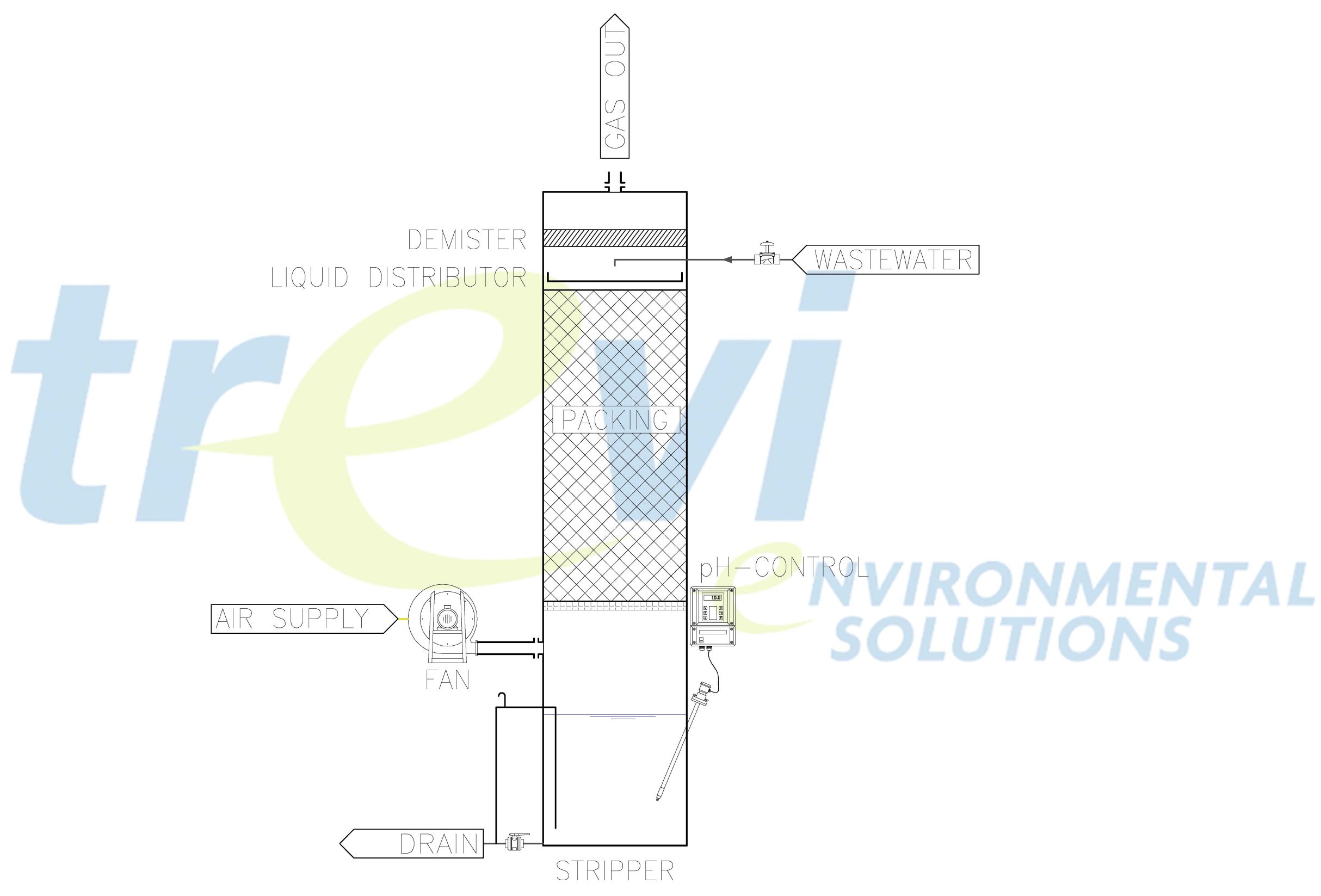
Principle
Stripping implies a transfer of volatile components from the liquid phase to the gas phase during an intensive contact in a stripping column filled with packing material (counter-flow principle).
For volatiles with a high vapour pressure, stripping can easily be obtained upon correct dimensioning of the stripping column and the gas flow. For other compounds as e.g. NH3, it is often needed to increase the pH and/or temperature of the scrubbing liquid in order to obtain optimal stripping efficiencies with acceptable air flows.
In most cases, a gas treatment system is required after the stripper. The outlet air of a NH3 stripper, for instance, can be sent to an acid scrubber and afterwards again recycled to the stripper in order to maintain the needed heat in the system and, as such, to minimise the energy cost.
Scheme
Realisations
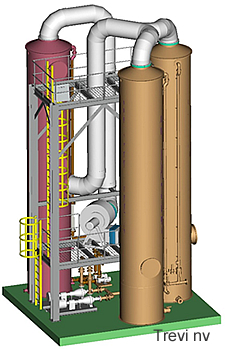
Loop of two NH3 strippers and an acid scrubber

ClO2 stripper